Here’s one way to harvest water right out of the air

Materials known as metal-organic frameworks, or MOFs, collect the moisture for drinking and other uses

Materials known as metal-organic frameworks can harvest humidity from the air, even when that air is relatively dry.
Water is something we cannot do without. People need it to grow crops and for a host of industrial processes. But we especially need it to drink. The body can’t survive more than three days or so without a drink of water. Now, researchers have developed materials that can pull water right out of thin air. It just might slake the thirst of those living in remote or dry areas.
The new materials belong to a type known as metal-organic frameworks (MOFs). Their name provides clues about what they’re made from. Imagine a set of super-tiny Tinkertoys. The hubs are small clusters of metal atoms. Chains of carbon-bearing compounds serve as the “sticks” linking those hubs together. When these components join up, they create an open, honeycomb-like structure.
MOFs have a range of useful traits. Last year, engineers reported getting green plants to take up the building blocks of MOFs and then assemble them internally. These MOFs were able to absorb harmful wavelengths of light so that they didn’t injure the plants. Other MOFs can filter toxic chemicals from the air and then store them or help break them down. Some can pull carbon dioxide from industrial smokestacks, which would help fight climate change. There are even MOFs that could be used in tanks to store hydrogen for electricity-making fuel cells. A system that used these would be lighter weight and safer to use, especially in vehicles.
But communities in arid parts of the world would be most interested in the MOFs that absorb water. These can capture plenty just from the air, says Zhiyong Xia. This materials scientist works at the Johns Hopkins University Applied Physics Laboratory in Laurel, Md.
Water molecules (H2O) are the perfect size and shape to pass through pores in the new MOFs. That lets them soak into the material. They have a second trait that’s just as important. Their internal arrangement of electrical charges attracts water.
A water molecule is somewhat V-shaped. A negatively charged oxygen atom sits at the bottom of that “V,” explains Xia. At the V’s upper tips sit two positively charged hydrogen atoms.
But not all water-collecting MOFs are equally useful, notes Xia. Some attract and latch onto water molecules too well. Later, you’d need a lot of energy to release any water collected by them. His solution: Identify a MOF that doesn’t hold onto water molecules so aggressively.
Now he and his colleagues have done just that.
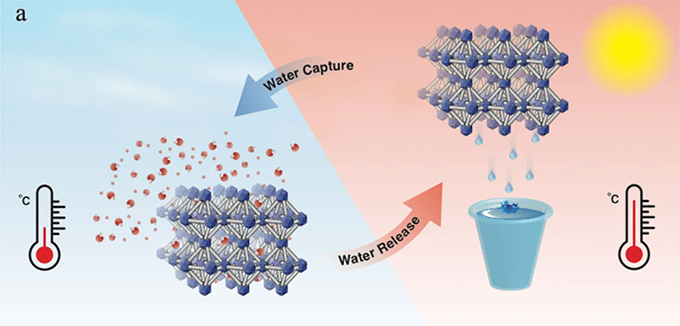
By absorbing humidity from cool air (left) and then releasing it at higher temperature, materials called metal-organic frameworks (MOFs) can collect water without using much energy.
Promise and potential
They investigated nine different MOFs that are able to harvest water. The metal in some was zinc. Others were made using titanium, copper, chromium or zirconium. In the lab, the researchers placed samples of each in humid, room-temperature conditions for a full day. Then, they heated the material in drier air to release the water that each had picked up. They repeated these cycles of soak-and-release at least 10 times.
Some MOFs didn’t pick up much water. Others soaked up a lot at first, but later released only a fraction of it. In later cycles, the material also might absorb much less than it initially did. Explains Xia, that’s a sign such materials attracted water a little too well.
In the end, Xia reports, one zirconium-based MOF stood out. The test samples were always small, he notes. But if the weight of that MOF sample had been 1 kilogram (2.2 pounds), the material would have absorbed and then released more than 8 liters (2.1 gallons) each day. That beats any previous MOF-based water-collection system.
Xia and his teammates described their findings January 30 in Scientific Reports.
Omar Yaghi is a materials scientist at the University of California, Berkeley. There’s a big difference between lab tests and field studies, he notes. What’s more, he points out, “The challenge is not just to take up water from humid air, but to do it at low humidity, too.”
Harvesting water vapor from air with humidity levels of less than 50 percent “is very challenging,” Yaghi says. But in recent field tests, his team and others have shown that some MOFs show great promise in water harvesting, even in desert-like environments. Specifically, they were able to recover water during tests in California’s Mojave (Moh-HAH-vee) Desert. They exposed their aluminum-based MOF to outdoor air with humidity as low as 10 percent. Even in those very arid conditions, a kilogram of the MOF they used would have been able to collect 0.7 liters of water from the air. His team reported its achievement August 27, 2019 in ACS Central Science.
Need water but you have no access to rain, lakes or groundwater? Materials known as metal-organic frameworks could be used to slurp that water from the air, new data show.
This is one in a series presenting news on technology and innovation, made possible with generous support from the Lemelson Foundation.
